Unipolar LV lead threshold test
Tracing
Manufacturer Medtronic
Device CRT
Field Left ventricular pacing
N° 2
Patient
64-year-old man implanted with a triple-chamber defibrillator Concerto II CRT-D with positioning of a Medtronic 4195 LV lead in a small lateral vein; complex implantation due to the small caliber of the veins + high threshold values + several pacing sites with a phrenic nerve capture.
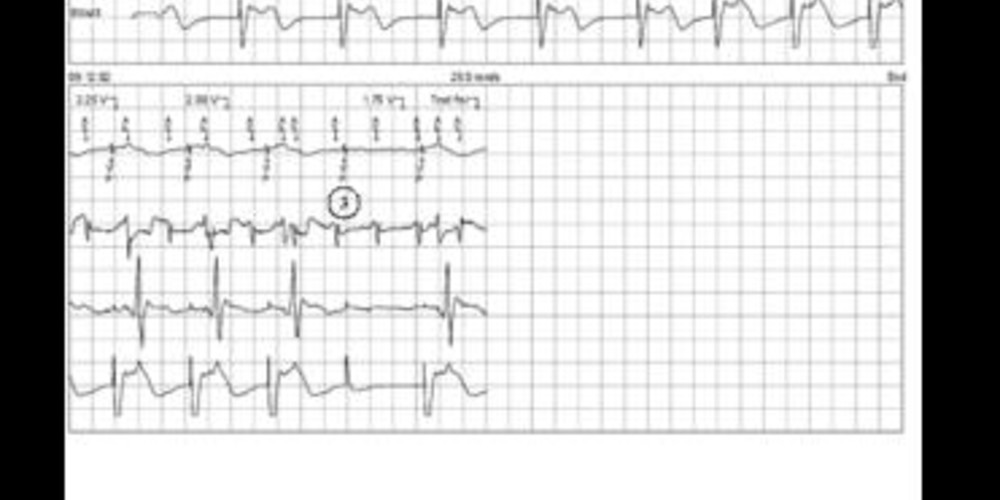
Graph and trace
- AF with biventricular pacing;
- LV thresholds (EGM3), LV tip – RV coil configuration;
- loss of LV capture (threshold 2.25 V/ 1.5 ms);
- LV threshold test in LV tip – LV ring (LV anode) configuration;
- absence of LV capture at maximal output (8 V/1.5 ms).
Other articles that may be of interest to you

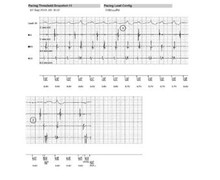
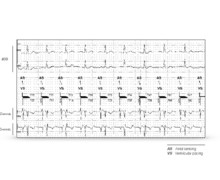




The armentarium of left ventricular leads proposed by the various manufacturers includes unipolar, bipolar and quadripolar leads. During interrogation of the device, it is essential to know the characteristics of the implanted material. Information pertaining to both patient and material (age, attending cardiologist, implantation indication, implanted lead models, etc.) are programmed into the device at the time of implantation. When a left ventricular unipolar lead is implanted and connected to a triple-chamber defibrillator, only one configuration (LV tip – RV coil) is programmable in the long term. Indeed, the pulse generator of a MedtronicTM defibrillator cannot be an integral part of the pacing circuit. On the other hand, it is possible, as in this example, to perform a threshold measurement in the LV tip - LV ring (anode) bipolar configuration. Pacing is obviously ineffective with, in dependent patients, the presence of a pause during the test. On a triple-chamber pacemaker, on the other hand, it is possible to program long-term unipolar pacing between the distal electrode and the can. Proper knowledge of the type of implanted material is essential at the time of generator change, especially given the advent of new connections for right ventricular leads (DF1 versus DF4) and left ventricular leads (IS1 versus IS4), so as not to de-sterilize an incompatible device.