Crosstalk and ventricular oversensing
Tracing
Manufacturer Medtronic
Device CRT
Field Standard parameters
N° 17
Patient
68-year-old man, implanted with a triple-chamber defibrillator Concerto II CRT-D for ischemic cardiomyopathy with complete AV bloc and episodes of atrial arrhythmias; implanted with a true (dedicated) bipolar ventricular lead in the inter-ventricular septum; several episodes of dizziness.
Graph and trace
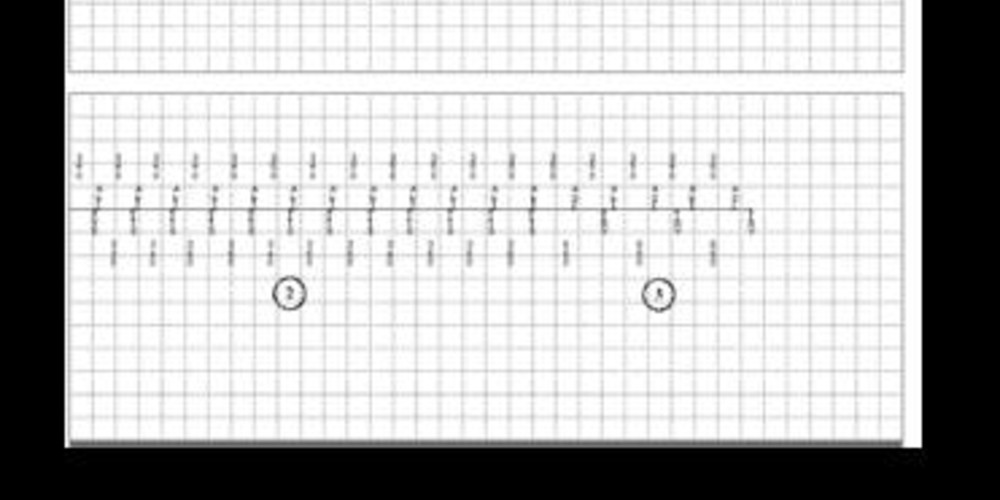
Episodes of ventricular sensed event in the device memory;
- possible atrial arrhythmia and biventricular pacing;
- spontaneous atrial and ventricular rhythm (with a 1:1 ratio and synchronous atrium and ventricle);
- recovery of biventricular pacing;
Episodes of non-sustained VT in the device memory - diagnosis of non-sustained VT;
- atrial arrhythmia and biventricular pacing; paced ventricular events are sensed in the atrium (VA crosstalk); we can also identify a small amplitude signal (non detected) on the ventricular channel preceding the atrial signals;
- probable AV crosstalk with sensing of the atrial signal by the ventricular channel and inhibition of biventricular pacing resulting in a pause of 2 to 3 seconds; the atrial component previously present but not sensed at the ventricular level, is now sensed; these are classified TS (in the VT zone);
- recovery of biventricular pacing;
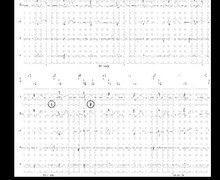
During this consultation, the complete interrogation of the device was performed with a programmed ventricular sensitivity of 0.5 mV.
EGM1: atrial EGM, EGM2: ventricular EGM (bipolar channel), EGM3: ventricular EGM (far-field channel) - atrial arrhythmia and biventricular pacing;
- programming change (increase in ventricular sensitivity to 0.3 mV);
- AV crosstalk; oversensing of the atrial activity by the RV lead during respiratory movements (breath); ventricular pause (false diagnosis of non-sustained VT);
- biventricular pacing recovery;
- programming of ventricular sensitivity at 0.5 mV;
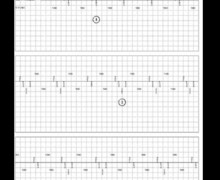
- atrial arrhythmia and biventricular pacing;
- activation of the response algorithm to sensed event (trigger pacing) and of ventricular sensitivity of 0.3mV;
- ventricular oversensing occurring during a deep breath but no pause since the biventricular pacing response to a sensed ventricular event is activated (VVT); markers are difficult to visualize since they are superimposed (fusion of VS and BV); this particular type of sensing is limited in frequency; however the device is less sensitive after a pacing than after a sensed event; accordingly, the first signals following the ventricular pacing are not sensed (sensing occurs at the end of the cycle when the sensitivity is maximal), which explains the limited frequency of intervention;
- interruption of oversensing;
- new episode of ventricular oversensing due to respiratory movements.
Other articles that may be of interest to you





This dependent patient had presented multiple episodes of lipothymia in the setting of a crosstalk between atrial arrhythmic activity and the ventricular canal inducing inhibition of ventricular pacing and a cardiac pause. This oversensing varied in conjunction with the respiratory cycles, appearing at inspiration and inducing only short pauses explaining the modest symptomatology.
There is no post-sensed atrial ventricular blanking period that can protect the device against this type of ventricular oversensing. In addition, the atrial signal was first seen by the ventricular channel then by the atrial channel, which would have canceled the effectiveness of such blanking.
Two options may be preferred in this setting: 1) repositioning of the ventricular lead; in this patient the lead was positioned at the level of the septum without visible dislodgement on chest X-ray; 2) finding a programming compromise; it is possible to change the right ventricular sense vector and to program an integrated bipolar ventricular sensing between the coil and the distal electrode. This option did not eliminate the oversensing, which is not surprising in the context of P-wave oversensing. The programming of the response to sensed events represents a second option and allows avoiding, as in this tracing, ventricular inhibition without suppressing oversensing. The third option consists in reducing ventricular sensitivity, which increases the risk of undersensing of a true ventricular arrhythmia. A reprogramming of ventricular sensitivity to 0.6 mV eliminated these oversensing episodes. A VF induction procedure was performed to verify the proper sensing of ventricular arrhythmia despite the programming change in ventricular sensitivity.